For this lab, we were introduced to Geneious, a program that performs a variety of functions on DNA and protein sequence data.
To begin, we downloaded our DNA barcoding sequences into Geneious. Using these sequences, we followed a tutorial protocol to familiarize ourselves with useful features of the program. This included: HQ% scores (percentage of bases that have a high quality score); analyzing the chromatogram view of our sequences (base letters are written in different sizes to match the height of the fluorescent colored peak above it); analyzing quality numbers associated with each base call (higher quality numbers are better); analyzing base call quality associated with color for each base (the darker the blue, the poorer the quality).
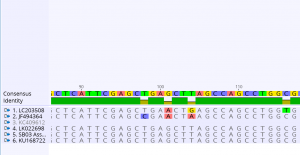
Next, we assembled sample forward (labeled _Fbc-F_) and reverse (labeled _Fbc-R_) sequences of the same sample. Using the sample, we followed a tutorial that outlined the steps to assemble, edit, and BLAST the sequence. The first step was De Novo assembly. Once the assembly was complete, we edited the sample sequences. Here, bases on both ends of the forward and reverse sequences were deleted if they were unreadable. We also looked for any ambiguities (N, Y, R, etc.) and manually edited the sequences at these sites. Then, we selected for “Generate Consensus Sequence.” Using this file, we performed BLAST (Basic Local Alignment Search Tool) on the strands. With this tool, we were able to identify the scientific name of the organism. Utilizing the same sequences and an additional 5 hits, we built an alignment. This allowed us to compare the sequences with the consensus identity and look for any polymorphic sites.
The above steps were applied to our individual samples that were cleaned up (see Lab 5); this included mh01, mh02, and mh04. However, samples 1 and 4 yielded unsuccessful barcode results. Thus, these samples were replaced with another students’ samples: sb02 (replaced mh01) and sb03 (replaced mh04).
After performing BLAST on sample mh02, the description listed seriola quinqueradiata. The common name that matches this species is Japanese amberjack. The restaurant listed this sample as yellowtail.
Seriola quinqueradiata was also listed for sample sb02. Like the previous sample, the common name is Japanese amberjack. For sample sb03, the scientific name was sparus aurata, with the common name being gilt-head bream. For the latter two samples, it is unclear what the fish was listed as from the restaurant it was sampled from.
Finally, alignments were assembled for each of the three samples. 42 polymorphic sites were found for sample mh02, 35 polymorphic sites were found for sample sb02, and 87 polymorphic sites were found for sample sb03.