Carter Pope
Professor John Paul
Molecular Ecology
10/9/18
Lab 6 Entry
An Introduction to Geneious
On the 3rd of October, I continued to work on the sushi project in the lab. I received my DNA barcoding sequences for my fish from Canvas and began the using the program called Geneious. Once Geneious was installed into my laptop and my DNA barcoding sequences were downloaded into Geneious, I began going through the tutorial handout on how to use the program. I learned how to do many tasks, such as creating new folders, reading forward/reverse sequences, and “BLASTING” my sequences.
From Exercises listed in the handout:
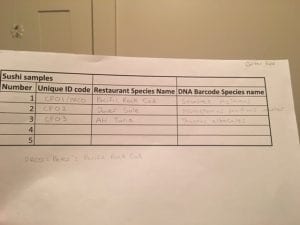
- Since my first fish sample (Pacific Rock Cod) didn’t work during the first round of the PCR, I used Peter Dubois’ Pacific Rock Cod sample (since we got the fish at the same grocery store) and discovered that we had a 99.4% pairwise value with Sebastes mystinus. Sebastes mystinus’ common name is the Blue rockfish. The description of the fish, which was Pacific Rock Cod, technically is an appropriate name for the fish sample because Blue rockfish falls under the category of Pacific rockfish, aka Rock cod. The second fish sample (Dover Sole) had a 99.5% pairwise value with Microstomus pacificus voucher. Microstomus pacificus voucher’s common name is Dover Sole and matched the species that was labeled in the grocery store. The third and final fish sample (Ahi Tuna) had a 99.8% pairwise value with Thunnus albacares. Thunnus albacares’ common name is yellowfin tuna. Both yellowfin tuna and bigeye tuna are the two species that are classified under ahi tuna, so technically my fish sample was correctly labeled in general.
2. For PRCD (Peter’s Pacific Rock Cod and Sebastes mystinus), there were four polymorphic sites in the alignment of the two located on the columns: 6, 9, 573, 615.
For CPO2 (Dover Sole and Microstomus pacificus voucher), there were three polymorphic sites in the alignment of the two located on the columns: 161, 203, 596.
For CPO3 (Ahi tuna and Thunnus albacares), there was one polymorphic site in the alignment of the two located on the column: 3.
Thoughts: I didn’t have more than ten polymorphic sites in my alignments which could suggest that my sequences were very short and if I had captured a longer sequence with no cuts previously made, I may have seen more polymorphic sites.
I then made a new alignment with included my three samples (including Peter’s PRC0) and 25 other sequences of different Actinopterygii that had the COI gene and had a similar length of around 500-700 bp, as well as an additional Chondrichthyes which acted as an outgroup for my phylogenetic tree that will be created next Wednesday on the 10th of October.